‘Is Life in Equilibrium?’ is Part Six of our ‘Life’ Series
In this post, Is Life in Equilibrium?, we continue our series on “Life” and our discussion of the ideas in Andy Pross‘s recent book, “What is Life?”.
Pross introduced us to the idea that life’s organized complexity represents a thermodynamic puzzle. But related to that complexity is another aspect of life‘s nature that is troubling with regards the Second Law of Thermodynamics – this is life’s far-from-equilibrium state.
The Hovering Bird
Pross gives the example of a bird hovering in the air, maintaining an almost stationary position by flapping its wings. The bird is in an unstable state. In order to maintain its hovering, and not drop to the ground, it must expend energy to flap its wings. By constantly flapping its wings, the bird is able to push air downwards to balance the pull of gravity on its body mass.

Maintaining a Cell State Needs Energy
The hovering bird is just one example of life’s far-from-equilibrium state; Pross goes on to describe the energetics of a bacterial cell. The cell is also unstable from thermodynamic point of view as the bacterial cell must constantly expend energy to maintain its cellular state.
Dissolving Table Salt in Water
He quotes the example of the existence and maintenance of ion concentration gradients in living cells. If you were to dissolve some table salt, NaCl, in water, the crystals of salt break up into sodium ions, Na+, and chlorine ions, Cl-. Initially, the concentration of these ions would be greatest where the salt crystals were introduced into the water. But gradually the ions would distribute themselves evenly throughout the volume of water by the natural action of diffusion and an equilibrium would be reached.
Another Example of the Second Law of Thermodynamics
This is another example of the Second Law where an initially unstable system (salt ions concentrated at the site of the introduction of the salt crystals into the water) becomes a stable system (salt ions evenly distributed by diffusion throughout the whole volume of liquid).
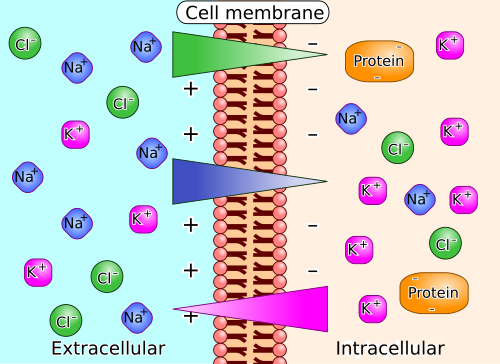
Ion Concentration Gradients
In contrast, living cells have inherently unstable ion concentration gradients that are essential for many physiological functions. The difference in ion concentration between the interior and exterior of a cell is termed an ion concentration gradient. This gradient is maintained over time by the cell in defiance of the Second Law.
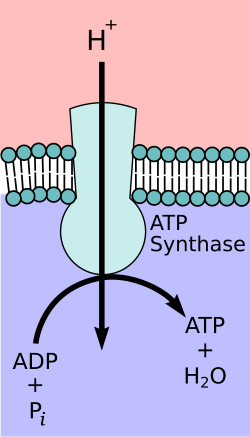
Gradients Need Ion Pumps
Pross asks, how can that be? He then explains that the cell has to operate ion pumps against the gradient – just like the bird must flap its wings to hover against the pull of gravity. The cell supplies and expends energy (e.g. using ATP, etc) to operate these ion pumps.
Ion Pumps Need Energy
There is no thermodynamic mystery in the ability of the cells to maintain that far-from-equilibrium state – they do so by continually expending energy which they extract from the environment. However there is the mystery of how the far-from-equilibrium chemical systems came into existence.
How Did Biological Far-from-equilibrium Systems Emerge from Chemistry?
We believe that chemical processes led to the emergence of life on earth. But chemical processes on the pre-biological earth would have been driven to an equilibrium state, to low energy chemical systems, in accordance with the Second Law.
How Did Biological Far-from-equilibrium Systems Evolve from Chemical Processes?
How, therefore, could pre-biological earth chemical processes that are driven to an equilibrium state have led to the emergence of complex, high-energy, far-from-equilibrium systems required for life? How was biology on earth born?
The Wikipedia article on Abiogenesis provides a detailed explanation of the evolution of present day biology from chemistry since the first life appeared on earth. This area of study is both extremely interesting and complex. Please note the question marks associated with the protocells in the diagram below:
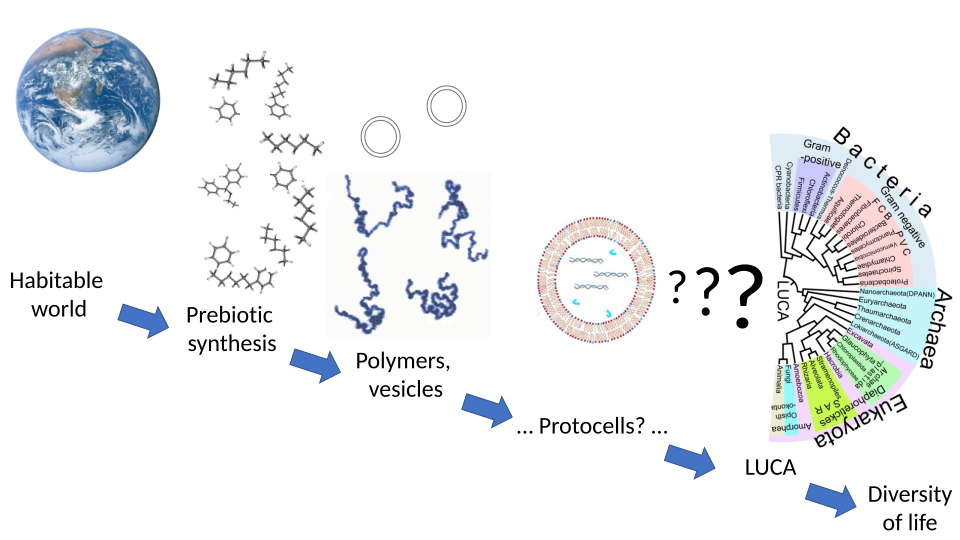
From Second Law Stability to Energetic Biological Imbalance?
The Second law states that all systems seek to become more stable, but this is exactly opposite to what must have occurred in order to produce far-from-equilibrium systems, that is the cellular biological systems that are in permanent imbalance, and that make up living things .
Pross states: “you can’t get there from here. But we did!” The question then is how did we?
Terraforming and far-from-equilibrium systems
This question of how life on earth came into being on earth is, of course, not an issue when it comes to terraforming a sterile planet and seeding it with existing life from earth’s biosphere. It is nevertheless an important question, and its answer may well throw light onto the other questions relating to creating a terraform biosphere.